Targeted and Advanced Therapies
in Pyruvate Kinase Deficiency
Targeted and Advanced Therapies in Pyruvate Kinase Deficiency
Who is affected: Adults with PK deficiency
Why it matters:
New approaches for treating people with PK deficiency are becoming available. Mitapivat, a drug that activates pyruvate kinase, is approved for the treatment of adults with PKD in the U.S., Great Britain, and E.U. The expert panel developed guidelines from research literature available as of 2023, on targeted and advanced therapies. Gene therapy trials are underway to test whether this treatment is safe and can cure children and adults with PK deficiency. Due to the limited available data for gene therapy, this therapy was not included in the guidelines.
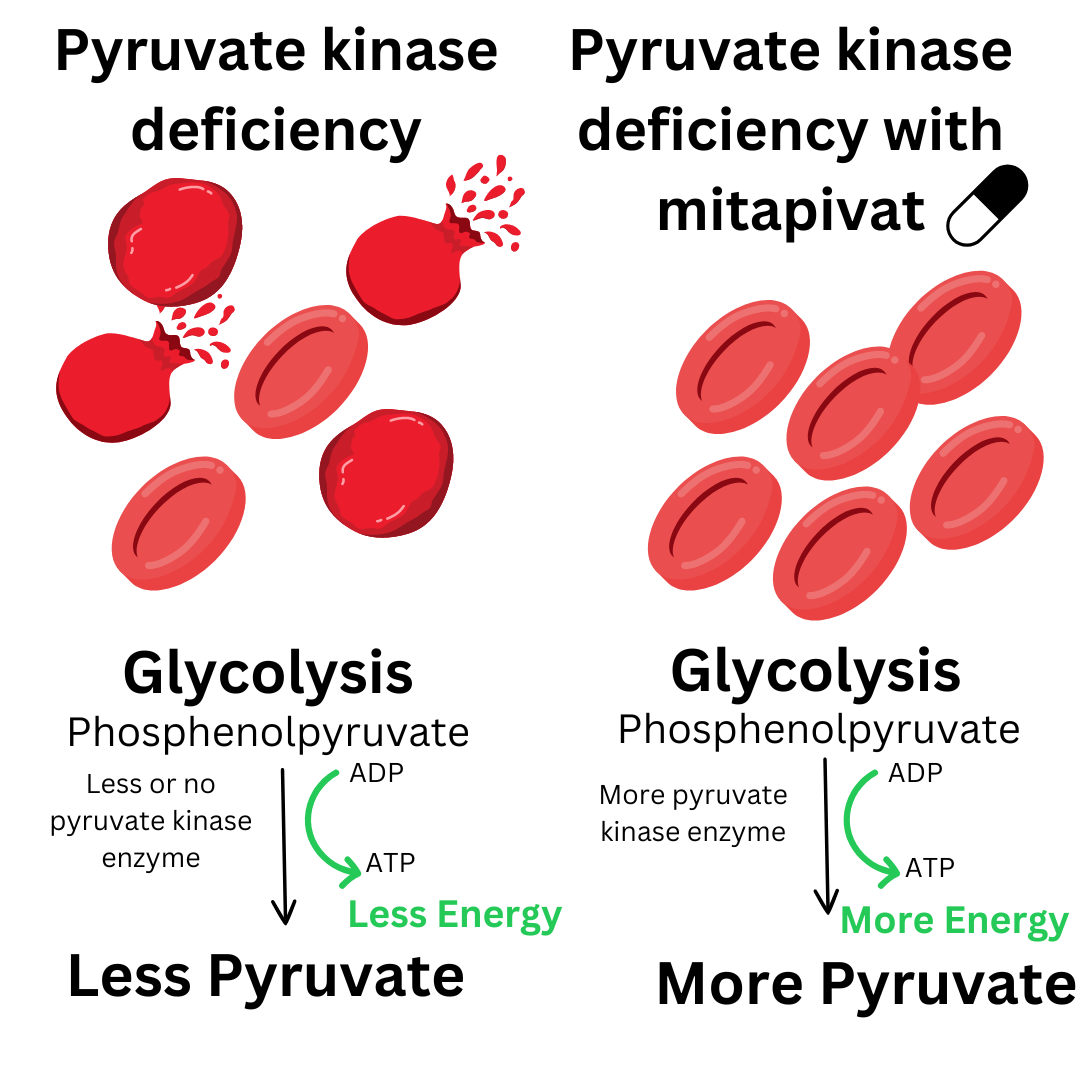
Flowchart for determining novel treatment use:
Initiate mitapivat use:
- Adults with PK deficiency who are anemic, not regularly transfused, do not have two non-missense mutations, regardless to splenectomy status, to improve hemoglobin and quality of life (Recommendation D1).
- Adults with PK deficiency who are regularly transfused, do not have two non-missense mutations, irrespective of splenectomy status, to reduce transfusion status (Recommendation D3)
- Adults with PK deficiency who are regularly transfused and have not undergone splenectomy prior to consideration of splenectomy (Recommendation D7)
Mitapivat nonresponder:
- Failure to respond to mitapivat should be declared only after 3 months of treatment at an optimal dose (Recommendation D2)
- Discontinue mitapivat in patients with PK deficiency who are non-responders, regardless of their transfusion status (Recommendation D4).
- Discontinue mitapivat in patients receiving regular transfusions who do not achieve at least a 33% reduction in transfusion requirement except for patients who received a marked improvement in iron status, patient-reported health outcomes, jaundice, or other key disease parameters (Recommendation D6).
Mitapivat responder:
- Response can be evaluated by improvement in hemoglobin and/or reduction in transfusions
- PK deficiency-specific measures of health-related quality of life (patient-reported outcomes) can be an indicator of success in cases when reduction in transfusion burden or increase in hemoglobin falls short (Recommendation D8)
Alternatives to mitapivat:
- Return patients who are non-responders to best supportive care (Recommendation D4)
- Consider alternative approaches, including clinical trials (Recommendation D5)
Bottom line:
Novel therapies are available for adults with PK deficiency and guidelines are set to ensure the best possible success rate based on patient-reported outcomes. People using new therapies for treatment are encouraged to actively monitor quality of life indicators to determine whether or not these options work for them.
